Human immunodeficiency virus (HIV) type 1 Vpu is an integral membrane protein with a singular affinity for betaTrCP (TrCP), a key member of the SkpI-Cullin-F-box E3 ubiquitin ligase advanced that’s concerned in the regulated degradation of mobile proteins, together with IkappaB.
Remarkably, Vpu is immune to TrCP-mediated degradation and competitively inhibits TrCP-dependent degradation of IkappaB, leading to the suppression of nuclear factor (NF)-kappaB exercise in Vpu-expressing cells. We now report that Vpu, via its interplay with TrCP, potently contributes to the induction of apoptosis in HIV-infected T cells. Vpu-induced apoptosis is restricted and impartial of different viral proteins.
Mutation of a TrCP-binding motif in Vpu abolishes its apoptogenic property, demonstrating a detailed correlation between this property of Vpu and its potential to inhibit NF-kappaB exercise.
The involvement of NF-kappaB in Vpu-induced apoptosis is additional supported by the discovering that the ranges of antiapoptotic factors Bcl-xL, A1/Bfl-1, and TNF receptor-associated factor (TRAF)1, all of that are expressed in an NF-kappaB-dependent method, are lowered and, at the identical time, ranges of lively caspase-Three are elevated. Thus, Vpu induces apoptosis via activation of the caspase pathway by approach of inhibiting the NF-kappaB-dependent expression of antiapoptotic genes.
The Caenorhabditis elegans Skp1-related gene household: various capabilities in cell proliferation, morphogenesis, and meiosis
BACKGROUND
The SCF ubiquitin-ligase advanced targets the ubiquitin-mediated degradation of proteins in a number of dynamic mobile processes. A key SCF element is the Skp1 protein that capabilities inside the advanced to hyperlink the substrate-recognition subunit to a cullin that in flip binds the ubiquitin-conjugating enzyme. In distinction to yeast and people, Caenorhabditis elegans incorporates a number of expressed Skp1-related (skr) genes.
RESULTS
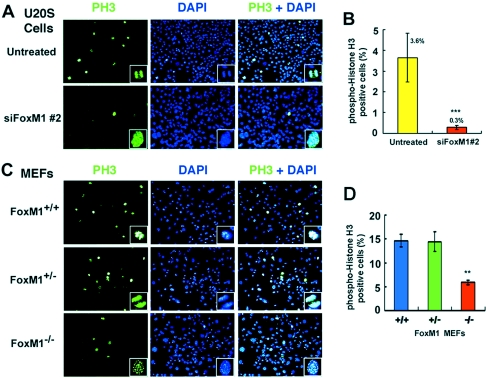
The 21 Skp1-related (skr) genes in C. elegans kind one phylogenetic clade, suggesting {that a} single ancestral Skp1 gene underwent impartial growth in C. elegans. The mobile and developmental capabilities of the 21 C. elegans skr genes had been probed by dsRNA-mediated gene inactivation (RNAi). The RNAi phenotypes of the skr genes fall into two lessons. First, the extremely related skr-7, -8, -9, and -10 genes are required for posterior physique morphogenesis, embryonic and larval growth, and cell proliferation.
Second, the associated skr-1 and -2 genes are required for the restraint of cell proliferation, development via the pachytene stage of meiosis, and the formation of bivalent chromosomes at diakinesis. CUL-1 was discovered to work together with SKR-1, -2, -3, -7, -8, and -10 in the yeast two-hybrid system. Interestingly, SKR-Three may work together with each CUL-1 and its shut paralog CUL-6.
CONCLUSIONS
Members of the expanded skr gene household in C. elegans carry out important capabilities in regulating cell proliferation, meiosis, and morphogenesis. The discovering that a number of SKRs are capable of bind cullins suggests an intensive set of combinatorial SCF complexes.
Nrf2 (nuclear factor erythroid 2-related factor 2) is a transcription factor that prompts transcription of a battery of cytoprotective genes by binding to the ARE (antioxidant response ingredient). Nrf2 is repressed by the cysteine-rich Keap1 (kelch-like ECH-associated protein1) protein, which targets Nrf2 for ubiquitination and subsequent degradation by a Cul3 (cullin 3)-mediated ubiquitination advanced.
We discover that modification of Cys(151) of human Keap1, by mutation to a tryptophan, relieves the repression by Keap1 and permits activation of the ARE by Nrf2. The Keap1 C151W substitution has a decreased affinity for Cul3, and might not serve to focus on Nrf2 for ubiquitination, although it retains its affinity for Nrf2.
A sequence of 12 mutant Keap1proteins, every containing a unique residue at place 151, was constructed to discover the chemistry required for this impact. The sequence reveals that the extent to which Keap1 loses the potential to focus on Nrf2 for degradation, and therefore the potential to repress ARE activation, correlates properly with the partial molar quantity of the residue.
Other physico-chemical properties don’t seem to contribute considerably to the impact. Based on this discovering, a structural mannequin is proposed whereby massive residues at place 151 trigger steric clashes that result in alteration of the Keap1-Cul3 interplay. This mannequin has vital implications for the way electrophiles which modify Cys(151), disrupt the repressive perform of Keap1.