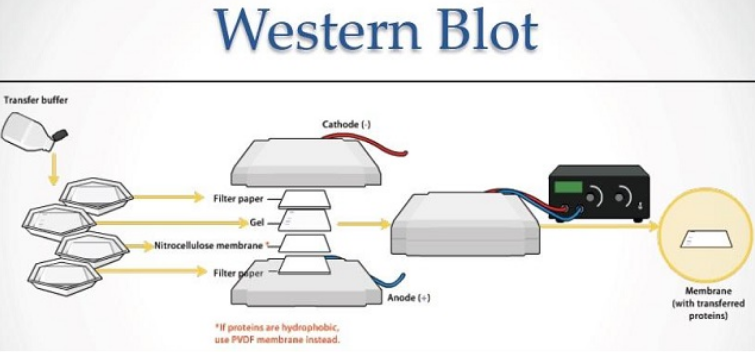
The Western Blot technique , also known as an immunoblot, is an immunoassay commonly used to identify, by means of antibodies , proteins previously separated by electrophoresis.
In this post we bring you a short guide with tips for solving problems in Western Blot , based on the most common causes.
How To Troubleshoot Western Blot
The most frequent problems usually translate into one or more of the following aspects: high background noise, weak or non-existent signal, multiple or diffuse bands, uneven staining of the gel and / or irregular and uneven spots throughout the blot.
Below we detail the possible causes (PC) as well as the tips or solutions (S) to solve them:
1.-High Background Noise :
PC: Antibody concentration too high
- S: Optimize and reduce antibody concentration
PC: Formation of aggregates of the secondary antibody
- S: Filter the secondary antibody or use a new one
PC: Antibody incubation temperature too high
- S: Incubate the antibody at 4ºC
PC: Nonspecific binding of the secondary antibody or cross-reactivity with the blocking agent
- S1: Run control of secondary antibody (without primary)
- S2: Reduce the concentration of the secondary antibody
PC: Cross reactivity of primary or secondary antibody with blocking agent
- S: Add Tween-20 to the incubation and wash buffers
PC: Incompatible blocking agent
- S: Compare different blocking buffers
PC: Incomplete crash
- S1: Optimize the choice of the blocking buffer
- S2: Increase the protein concentration in the blocking buffer
- S3: Optimize blocking time and / or temperature
- S4: Add Tween-20 to the blocking agent
PC: Insufficient lock
- S: Extend the blocking time or use a compatible blocking agent
PC: Cross reactivity of the antibody with other proteins
- S1: Use different blocking agents
- S2: Reduce the concentration of the secondary antibody
- S3: Analyze the cross reactivity between the secondary antibody and the membrane
PC: insufficient washing
- S1: Increase the number of washes and the volume of the buffer
- S2: Add Tween-20 to the wash buffer
PC: Exposure time too long
- S: Reduce exposure time
PC: Problems with the membrane
- S1: Use a new membrane
- S2: Make sure the membrane stays moist
- S3: Handle carefully avoiding damage to the membrane
PC: Incompatible membrane
- S: In general, nitrocellulose membranes tend to give less background noise
PC: Contaminated buffer
- S: Filter the buffer or use a new one
2.- Weak Or Non-Existent Signal
PC: Incorrect transfer of protein to membrane
- S1: Use Ponceau to check the transfer efficiency
- S2: Ensure the correct contact between the membrane and the gel during the transfer
- S3: Moisten the membrane according to the instructions
- S4: Avoid overheating during transfer
- S5: Use positive controls or molecular weight markers
- S6: Optimize transfer time and current
PC: Insufficient bond between protein and membrane
- S: Add methanol to the transfer buffer
PC: Insufficient antibody
- S: Increase antibody concentration
PC: Insufficient antigen
- S: Load more protein
PC: Masking of antigen by blocking buffer
- S1: Compare different blocking buffers
- S2: Optimize protein concentration of blocking buffer
- S3: Reduce blocking time
PC: Presence of sodium azide in buffers
- S: Remove sodium azide from buffers
PC: Exposure time too short
- S: Increase the exposure time
PC: Substrate incubation time too short
- S: Increase incubation time
PC: Digestion of the protein in the membrane
- S: Optimize the amount of blocking agent
PC: Degradation of protein during storage
- S: Re-prepare the sample with the protein
PC: Incompatibility between primary and secondary antibody
- S1: Make sure compatibility
- S2: Use loading controls for Western Blot to check the effectiveness of the secondary antibody (You can consult the guide on “How to select loading controls for western blot” here )
PC: Low concentration of primary and / or secondary antibody
- S1: Increase the concentration of antibody
- S2: Increase incubation time
PC: Cross reactivity between blocking agents and antibodies
- S1: Add Tween 20
- S2: Change the blocking agent
PC: Inability of the primary antibody to recognize the protein
- S1: Review the instructions for use
- S2: Use positive controls
PC: Not enough protein
- S1: Increase the amount of charge in the gel
- S2: Use protease inhibitors
- S3: Use positive controls
PC: Insufficient transfer and excessive washing
- S1: Confirm the transfer with Ponceau
- S2: Avoid excessive washing
PC: excess lock
- S1: Change the blocking agent
- S2: Reduce blocking time
PC: Inhibition of the secondary antibody by sodium azide
- S: Avoid co-use of sodium azide with HRP-conjugated antibodies
PC: Methanol concentration too high
- S: Reduce the concentration of methanol or use isopropyl alcohol
PC: Idle Conjugate
- S: Mix the enzyme and the substrate in a tube to confirm that a colorimetric reaction occurs
3.- Multiple Bands
PC: The protein in the sample has been digested
- S1: Make sure that enough protease inhibitor has been added to the buffer
- S2: Avoid sample freeze / thaw cycles
PC: Nonspecific binding of the primary antibody
- S1: Reduce the concentration of primary antibody
- S2: Reduce the amount of protein loaded in the gel
- S3: Purify the antibody by affinity with the antigen
PC: Too much protein per lane or too sensitive detection system
- S: Make serial dilutions of the starting material
PC: Ineffective lock
- S: Modify blocking conditions
PC: Antigen concentration too low
- S: Try to enrich the antigen by fractionation or immunoprecipitation
PC: Nonspecific binding of the secondary antibody
- S1: Run a secondary antibody control, without the primary
- S2: Select another secondary antibody (You can consult the guide to select secondary antibodies here )
- S3: Adjust the antibody concentration
- S4: Increase the number of washes
- S5: Use monospecific antibodies
PC: Analyte forms aggregates
- S1: Increase the amount of DTT
- S2: Heat in a water bath before loading the gel
- S3: Centrifuge briefly
PC: Contamination of reagents
- S1: Check that the buffers do not contain particles or microbial contamination
- S2: Use new reagents
4.- Fuzzy Bands
PC: Excess protein in the gel
- S: Reduce protein load
5.- Uneven Staining Of The Gel
PC: Bacterial contamination
- S: Keep the antibodies at 4ºC and use fresh buffers (you can consult the guide on “How to store the antibodies” here )
PC: Insufficient volume of antibody
- S: Make sure the membrane is covered by the antibody and shake during incubation
6.- Irregular And Uneven Points Throughout The Blot
PC: Contamination of reagents
- S1: Check that the buffers do not contain particles or bacterial contamination
- S2: Use new reagents
PC: Insufficient volume of solution during incubation or washing
- S: Make sure that the membrane is completely immersed during the washes and incubations with the antibodies
PC: Air bubbles trapped in the membrane
- S: Eliminate bubbles, especially during transfer
PC: Irregular shaking during incubation
- S: Make sure the stirring is uniform
PC: HRP Aggregation
- S: Filter conjugates to remove aggregates
Western Blot is a technique that involves several steps, and there are no universal optimal conditions that can be applied to all samples. For this reason, there are a multitude of variables that may affect the results of the tests as expected.
We hope that these tips have been useful for solving problems in Western Blot . And if you still have any questions, do not hesitate to contact us.